Abstract
Background:
Platelets, derived from megakaryocytes primarily play a central role in thrombosis and hemostasis, however, they extend beyond this role as immune cells that initiate and accelerate various vascular inflammatory conditions. Upon activation, platelets release TREM-Like Transcript-1 (TLT-1) from their a-granules onto their surface. Early studies by amino link columns preloaded with soluble TLT-1 followed by mass spectrometry and immunoblotting identified fibrinogen as a ligand for TLT-1. Fibrinogen is a plasma protein that is essential for clot formation, during inflammation and hypercoagulable states tissue deposition and plasma concentration of fibrinogen are increased, demonstrating a role of fibrinogen in both thrombosis and inflammation.
TLT-1 binding fibrinogen was a surprising discovery since αIIbβ3, the most abundant platelet receptor, also binds fibrinogen and facilitates platelet aggregation. It is difficult to understand why there are two platelet specific receptors that have the same ligand, drawing us to question what the difference in function between the two is? Our studies suggest that although TLT-1 may assist in clot formation and hemostasis to arrest bleeding in a non-inflammatory setting like αIIbβ3, TLT-1's main association is with regulating inflammatory-derived bleeding. Very little is known about the TLT-1-Fibrinogen interaction, further studies would set the stage for a better understanding as to why two fibrinogen ligands exist on platelets and potentially outline a novel platelet therapeutic target during hypercoagulable and/or hyperinflammatory states. We set out to determine the binding affinity and localize the binding sites for the TLT-1 fibrinogen molecular interaction.
Aims:
Delineate the TLT-1 fibrinogen molecular interaction and elucidate the mechanism by which this interaction drives inflammation and thrombosis-hemostasis.
Methods:
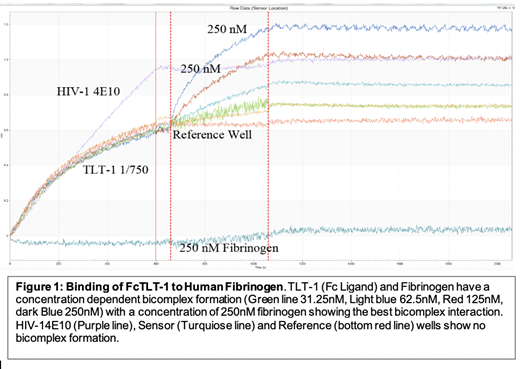
To confirm the TLT-1 fibrinogen ligand interaction we carried out a kinetics assay using an Octet Qk e Bio-layer Interferometry (BLI) that measures biomolecular complex formation in real time. The TLT-1 Chimera was captured onto an Anti-Human Fc Capture (AHC) Biosensor, washed in kinetics buffer to limit nonspecific binding and submerged in a 96 well plate containing varying concentrations of Fibrinogen. To localize the exact binding sites for this molecular interaction, we digested fibrinogen using trypsin and carried out an immunoprecipitation (IP) followed by Liquid Chromatography-Mass Spectrometry (LC-MS/MS).
Results:
The curve (Figure 1) shows that the TLT-1 fibrinogen interaction has increasing bimolecular complex formation with increases in concentration of Fibrinogen (15.625nM - 250nM), with a concentration of 250nM showing the best bicomplex formation. In the control well with HIV01 4E10 capture, reference and sensor well, no bicomplex formation is shown, highlighting the specificity of the TLT-1 fibrinogen interaction. The curve illustrates a strong association with no dissociation, suggesting a strong interaction between the proteins. We isolated and identified four potential peptides (Alpha chain: GGSTSYGTGSETESPR, GSESGIFTNTK, Beta chain: QDGSVDFGR , QGFGNVATNTDGK) that bind TLT-1. We are currently performing BLI Competitive kinetics assays using biotinylated constructs of the peptides isolated from the Immunoprecipitation/ LC-MS/MS. The BLI competitive assays using the four peptides are suggestive of an interaction between TLT-1 and the four peptides as illustrated by increasing bimolecular complex formation with increasing concentration of soluble TLT-1 for all four peptides(data not shown).
Conclusions:
We obtained an equilibrium dissociation constant (KD) of 3.02 ± 0.20 nM for the TLT-1 fibrinogen interaction, suggesting a high affinity interaction between TLT-1 and fibrinogen. In our preliminary results from the BLI Competitive kinetics assays we obtained KD values within the nanomolar concentration range and are currently conducting experiments to optimize conditions to obtain our final bicomplex binding curve and KD values. We are currently assessing the identified peptides for potential of mediating the molecular interaction between TLT-1 and fibrinogen. Our poster will report the current state of these studies .
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal